difference between osmosis and diffusion class 9|Diffusion and osmosis (video) : Baguio FAQs about Diffusion And Osmosis. Question 1: What is the difference between diffusion and osmosis in terms of speed? Answer 1: Diffusion is . Read 47,789 galleries with tag bondage on nhentai, a hentai doujinshi and manga reader.
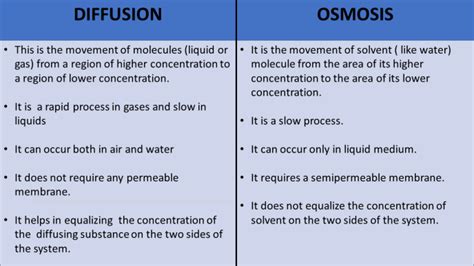
difference between osmosis and diffusion class 9,Osmosis can only function in a liquid medium, but diffusion can occur in all three mediums (solid, liquid and gas). Furthermore, osmosis requires a semi-permeable membrane, while diffusion does not. The intake of water in plants is an example of osmosis.Osmosis is the movement of solvent particles from a solution that is diluted to a more concentrated one. In contrast, diffusion is the movement of .FAQs about Diffusion And Osmosis. Question 1: What is the difference between diffusion and osmosis in terms of speed? Answer 1: Diffusion is .Diffusion and osmosis (video) There are key differences between osmosis and diffusion: Osmosis only occurs across a semipermeable membrane, while .
The difference between Osmosis and diffusion certainly exists. Each carries an equal amount of importance in our bodies. Lack of any of these can be very har.About. Transcript. Diffusion refers to the movement of molecules from an area of high concentration to an area of lower concentration. Osmosis is a type of diffusion .
Osmosis. It can be defined as the movement of water molecules from a higher water concentration area to the area of less water concentration through a .

Table of content. 1 Introduction to Difference Between Osmosis and Diffusion. 1.1 Uphill Concentration Gradient in Osmosis. 1.2 Downhill Concentration Gradient in Diffusion. 1.3 The variance of Moving .Biology Tutorials. Diffusion and Osmosis. Page ID. Katherine Harris. Hartnell College. 1. Description of Diffusion and Osmosis. A water solution that contains nutrients, wastes, gases, salts and other substances .
Now, after studying all the differences between osmosis and diffusion; we can conclude that options a.), b.) and c.) all three are the correct answers. Note: Diffusion does not require a semipermeable membrane while osmosis does require. When osmosis is a unidirectional phenomenon; the diffusion occurs in all directions. Difference Between Osmosis and Diffusion for Class 9. The Main Difference Between Osmosis and Diffusion is Osmosis can only work in a liquid medium, although diffusion can happen in any of the three (solid, liquid and gas). Difference between osmosis and diffusion: Osmosis and diffusion are two types of transport .The movement of any substance can move from higher concentration to the lowest. 2. Semi permeable membrane is needed. 2. Semi permeable membrane is not needed. 3. Osmosis requires liquid medium. 3. Diffusion can occur place in any medium.The movement of molecules takes place by various processes such as diffusion and osmosis. As we know, Diffusion is the movement of particles from a region of higher concentration to a region of lower concentration until the equilibrium is achieved. And Osmosis is the process of the passage of solvent molecules from a region of higher .Understanding the difference between osmosis and diffusion is a fundamental concept in Biology, especially for students in Class 9. These two processes play a crucial role in various biological phenomena, from the absorption of water by plants to .
3. In osmosis, diffusion of the only solvent from a lower concentration of solution to a higher concentration of solution occurs. 4. It is influenced by the diffusion pressure. 4. It is only influenced by the turgor pressure. 5. It is the process in which water molecules move through a semi-permeable membrane from a region of higher .Another difference between osmosis and diffusion is that the molecules vary. When diffusion takes place across a membrane, it is dependent on the molecule’s size as well as the electric charge. Similarly, the smaller the molecule, the faster it will diffuse when compared to larger molecules. On the other hand, the molecules which are charged .This response will provide a detailed explanation of the differences between osmosis and diffusion.Osmosis:Osmosis is a specific type of diffusion that involves the movement of water molecules across a semi-permeable membrane. . EduRev has designed Class 9 study material, with Structured Courses, Videos, & Test Series. Plus get personalized .1. In osmosis movement of molecules takes place through a semi-permeable membrane. 2. It involves movement of only solvent molecules from one side to the other. 3. Osmosis is limited to solutions only. 4. Osmosis can be stopped or reversed by applying additional pressure on the solution side. Diffusion. State the Difference between Osmosis and Diffusion for Class 9 : Osmosis and Diffusion are two different types of transport and both are completely different from each other. In this article we explain all their difference between Osmosis and Diffusion so that you can distinguish them perfectly in your preparation. Before .
Transport in Cells: Diffusion and Osmosis | Cells | Biology | FuseSchoolIn this video we are going to discover how cells take in useful substances and remove.The major difference between endosmosis and exosmosis are summarized below: The solvent moves into the cell. The solvent moves out of the cell. Osmosis towards the inside of a cell. Osmosis towards the .Transcript. Diffusion refers to the movement of molecules from an area of high concentration to an area of lower concentration. Osmosis is a type of diffusion specifically for water molecules moving across a semi-permeable membrane. A concentration gradient is the difference in concentration of a substance between two areas, which drives .Differerence between Osmosis and Diffusion. (Hindi) Transport in Plants for Class 11th. 21 lessons • 3h 25m. 1. Course Overview (in Hindi) 11:09mins. 2. Transport in Plants- Introduction Part 1 (in Hindi) 8:37mins.difference between osmosis and diffusion class 9 CBSE Class 9 chemistry Differences between Osmosis and Diffusion #osmosis ,#upsc,#Diffusion #ncert Difference between Osmosis and DiffusionOsmosisDiffusionIt.difference between osmosis and diffusion class 9 Diffusion and osmosis (video) CBSE Class 9 chemistry Differences between Osmosis and Diffusion #osmosis ,#upsc,#Diffusion #ncert Difference between Osmosis and DiffusionOsmosisDiffusionIt.Verified Answer. Differences between Osmosis and Diffusion. Osmosis: Osmosis is the movement of solvent particles across a semipermeable membrane from a dilute solution into a concentrated solution. The solvent moves to dilute the concentrated solution and equalize the concentration on both sides of the membrane. Examples of Osmosis:
Diffusion and Osmosis Questions and Answers - Practice questions, MCQs, PYQs, NCERT Questions, Question Bank, Class 11 and Class 12 Questions, NCERT Exemplar Questions, and PDF Questions with answers, solutions, explanations, NCERT reference, and difficulty level in Diffusion and Osmosis chemistry.

In this short video, you will learn about the difference between osmosis and diffusion. This concept is crucial for anyone who wants to become a great clinic. In this short video, you will learn about the difference between osmosis and diffusion. This concept is crucial for anyone who wants to become a great clinic.
difference between osmosis and diffusion class 9|Diffusion and osmosis (video)
PH0 · What Is the Difference Between Osmosis and Diffusion?
PH1 · Osmosis vs Diffusion
PH2 · Explore the Difference Between Diffusion And Osmosis
PH3 · Diffusion and osmosis (video)
PH4 · Diffusion and Osmosis
PH5 · Diffusion And Osmosis
PH6 · Difference between Osmosis and Diffusion
PH7 · Difference Between Osmosis and Diffusion in Tabular Form
PH8 · Difference Between Osmosis and Diffusion in Tabular
PH9 · 4. Diffusion and Osmosis Notes NCERT Solutions for CBSE